Having a range of COVID-19 vaccines available for people to use around the world will be essential to bringing the pandemic under control.
Here’s why (red arrow down)
*1. We need a range of vaccines that can work for a range of people*
We need COVID-19 vaccines to work for a diverse range of people – including older people and those with underlying health conditions. At this stage, we don’t have sufficient data to understand how effective the vaccines are for people of all ages, from different ethnic backgrounds, with different immune systems; and for different strains of the virus. We also don’t know how long immunity might last for, or whether the vaccines stop transmission of the disease.
With questions like these unanswered, we can’t rely on one or two vaccines to help us recover from COVID-19.
Vaccines are a bit like different painkillers: aspirin, paracetamol, and ibuprofen – they all have the same goal, they just achieve it in slightly different ways.
If we have a varied COVID-19 vaccine portfolio, we can be confident we will find the right vaccine for every person.
*2. We need to produce billions of vaccine doses to protect those most at risk*
To be able to get control of the virus we will need to produce and roll out vaccines at a scale and speed never seen before. To meet the aim of vaccinating high-risk populations around the world by the end of 2021(opens in a new tab), estimates suggest we need at least 2 billion vaccine doses.
Because many countries have pre-ordered large quantities of the vaccine, a large share of the initial production is already spoken for. Companies are scaling up production as much as they can, for example Moderna have stated they hope to produce 600 million doses of their vaccine in 2021
However, current models predict that there will not be enough vaccines to cover the world’s population until 2023 or 2024
We have also never licensed or scaled-up an RNA vaccine before – the technology that the Moderna and Pfizer-BioNTech vaccines use – and doing this requires specialised equipment. So there may be unforeseen manufacturing issues while new facilities and processes are being set up, potentially causing delays and limiting availability.
By developing and investing in multiple vaccine candidates we stand a much better chance of having the volume of doses we need to get the virus under control.
*3. We need vaccines that can reach everyone, everywhere*
We will need to get COVID-19 vaccines to everyone who needs them, wherever they are – from people living in metropolitan areas and cities, to rural populations in remote corners of the world.
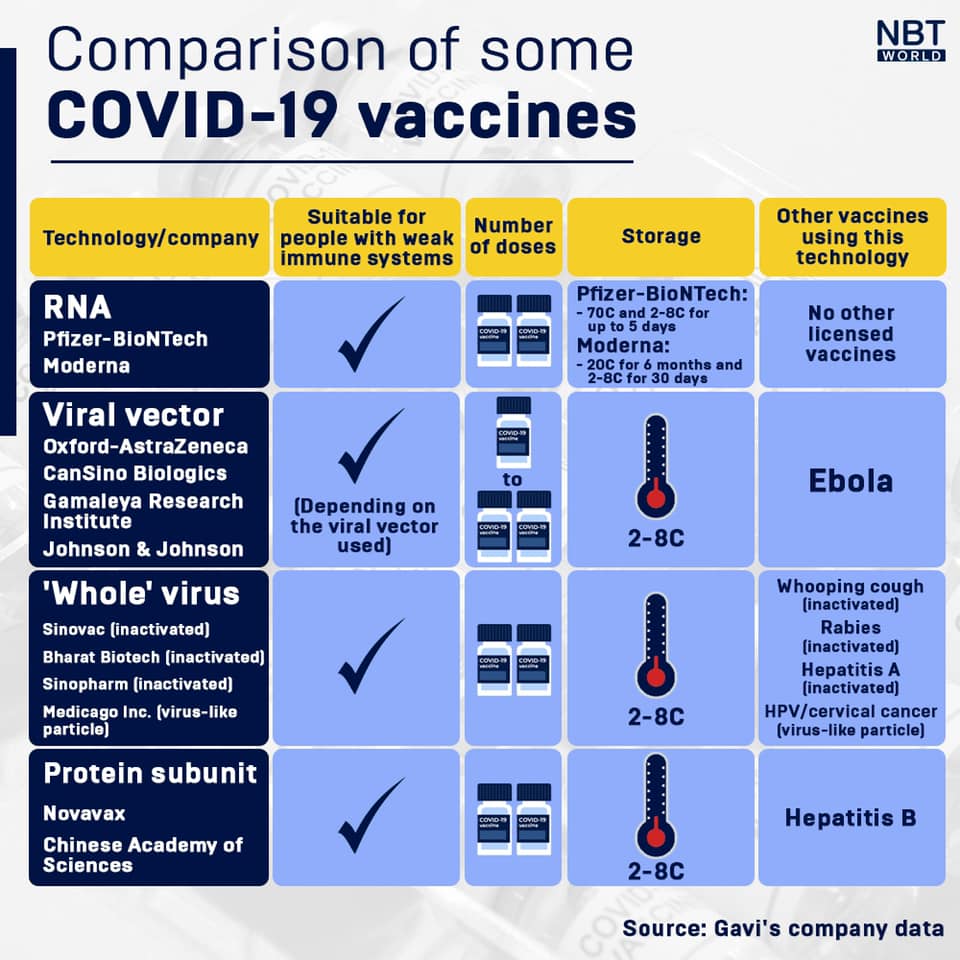
Both the Pfizer-BioNTech and Moderna vaccines need to be stored and transported at exceptionally low temperatures, the Pfizer-BioNTech vaccine at -70C and the Moderna vaccine at -20C, to ensure they stay viable. In comparison, most childhood vaccines require storage at between 2C to 8C, similar to the temperature in a domestic fridge.
The requirements for cold storage and transportation will be difficult to meet in many locations, and no vaccine requiring such cold storage has ever been rolled out to so many countries all at once.
In addition, both RNA vaccines currently have a relatively high cost per dose, although this should fall once large scale production and supply chains are in place. The news that the Oxford-AstraZeneca vaccine costs significantly less has been widely welcomed, but it is hoped that more highly effective vaccines will be offered at a lower cost.
Dosing creates an additional challenge. Most approved vaccines to date require two doses, spaced a few weeks apart. Developing effective single-dose vaccines will be critical to protect people for whom getting two doses might be difficult, such as those without access to regular healthcare, or refugees. Some manufacturers, including Johnson & Johnson, have developed single dose vaccines, and it could be a turning point in global vaccine access.
These technical considerations mean it is vital that we continue to develop and invest in multiple vaccine candidates, which may be able to offer high levels of efficacy without the need for such cold storage, or multiple doses. (NNT)





